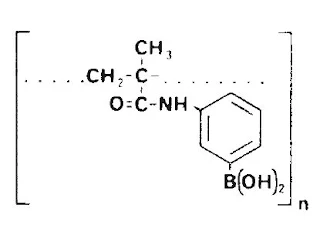
Description and Characteristics Boric Acid Gel is a cross-linked polymer insoluble in water and all organic solvents. It is prepared by the cross-linking copolymerization of dihydroxyborylanilino- substituted methacrylic acid with 1,4-butanediol dimethacrylate. The gel is swollen in distilled water, then activated with 0.5N HCl. It is then washed to neutral pH and vacuum-dried. Thus, the gel is supplied "activated," as a nearly free-flowing granulate.
 |
| Boric Acid Gel |
Appearance : nearly dry, off-white granules
Boron content : 1.4% (dry)
Packing volume : ca. 0.6g/mL
Degree of swelling : ca. 80% (i.e., final increase in volume ca. 20%)
Bead size : 0.1-0.4mm
Ribose-binding capacity :approx. 0.01mmol/mL (The Boric Acid Gel offered by Aldrich has
been shown to possess a binding capacity four times that reported
in the literature.
Boron content : 1.4% (dry)
Packing volume : ca. 0.6g/mL
Degree of swelling : ca. 80% (i.e., final increase in volume ca. 20%)
Bead size : 0.1-0.4mm
Ribose-binding capacity :approx. 0.01mmol/mL (The Boric Acid Gel offered by Aldrich has
been shown to possess a binding capacity four times that reported
in the literature.
Application
Boric Acid Gel serves as useful packing material for the column chromatographic separation of mixtures whose components form complexes of varying stability with boric acid. Separations of sugars and nucleic acids based on boric acid complex formation are standard procedures. However, preparative separations are very complicated because of the difficulty in separating the resolved components from the borate buffer used. The major advantage of the use of Boric Acid Gel is that the components are eluted from the column in the free, non-complexed form. The boric acid, due to covalent linkage to the polymer, remains in the stationary phase.
Boric Acid Gel serves as useful packing material for the column chromatographic separation of mixtures whose components form complexes of varying stability with boric acid. Separations of sugars and nucleic acids based on boric acid complex formation are standard procedures. However, preparative separations are very complicated because of the difficulty in separating the resolved components from the borate buffer used. The major advantage of the use of Boric Acid Gel is that the components are eluted from the column in the free, non-complexed form. The boric acid, due to covalent linkage to the polymer, remains in the stationary phase.
Separations of mono-and oligosaccharides,2 ribonucleosides- deoxyribonucleosides,1 oligonucleosides,3 and tRNA have been reported. Separation is dependent upon complex formation, and the pH and molarity of the buffer. Boric Acid Gel may be used in aqueous solution at pH 3-11 and in organic solvents.
References
(1) Schott, H. Angew. Chem., Int. Ed. Engl. 1972, 11, 824. [Angew. Chem. 1972, 84, 819].
(2) Reske, K.; Schott, H. Angew. Chem., Int. Ed. Engl. 1973, 12, 417. [Angew. Chem. 1973, 85, 412].
(3) Schott, H.; Rudloff, E.; Schmidt, P.; Roychoudhury, R.; Kössel, H. Biochemistry 1973, 12, 932.
(1) Schott, H. Angew. Chem., Int. Ed. Engl. 1972, 11, 824. [Angew. Chem. 1972, 84, 819].
(2) Reske, K.; Schott, H. Angew. Chem., Int. Ed. Engl. 1973, 12, 417. [Angew. Chem. 1973, 85, 412].
(3) Schott, H.; Rudloff, E.; Schmidt, P.; Roychoudhury, R.; Kössel, H. Biochemistry 1973, 12, 932.
This information is presented to assist you in evaluating product. It is intended for use by technically-skilled persons.
We do not guarantee favorable results, and we assume no liability in connection with its use.
We do not guarantee favorable results, and we assume no liability in connection with its use.
We are providing highly pure and utmost quality of uncoated Calcium Carbonate Precipitated series that meets the current standard and norms of polymer Industry’s requirement.
ReplyDelete👌👌👌👌👌👌👌
ReplyDelete