ACETYLENE INDUSTRIAL MANUFACTURING PROCESS
Reaction
RAW MATERIAL REQUIREMENT TO MAKE 1 METRIC TON OF ACETYLENE
Calcium Carbide (85%) : 3490 kg
Water :
28,825 kg
Calcium Carbide is formed when Lime
(essentially free of phosphates and Magnesium Carbonate) and Coke (with low ash
content), mixed in a ratio of 60:40 are heated to a temperature of 2000 to 2100
degree C in an electric furnace. The Carbon Monoxide may be recovered and used
as a fuel in lime kilns or as a raw material for chemical synthesis. The liquid
Calcium Carbide is allowed to cool and solidify for 24 to 48 hours and is then
crushed and screened for size. The grinding operation are carried out in an
atmosphere of Nitrogen to prevent explosion from Acetylene Generated by
moisture in the air. The approximate requirements per metric ton of Carbide
are:
Lime – 991 kg, Coke – 683 kg, Electrode
Paste – 17 to 20 kg, Electricity – 3200 kWh, and labor – 5 man hours.
The Carbide so produced is about 80%
Calcium Carbide; 1 kg of Carbide yields about 0.29 cubic meter of Acetylene.
These are two standard methods for the
production of Acetylene from Calcium Carbide and water: the wet process and the
dry process. The wet process consists of adding the carbide to a relatively
large quantity of water, releasing Acetylene gas while the calcium Hydrate
residue is discharged in the form of a Lime slurry containing approximately 90%
water.
In order to eliminate the waste of
Calcium Hydrate, the dry process was originated in which a limited amount of
water (1:1 by weight) is added to Calcium Carbide in a generator. The heat of
reaction (1475 kcal/cubic meter) Acetylene generated) is employed to vaporized
the excess water over the chemical equivalent, leaving a substantially dry
Calcium Hydrate suitable for reuse as a Lime source. The temperature must be
carefully controlled, since Acetylene Polymerized to from Benzene at 600 degree
C and violently decomposes at 780 degree C, and Air mixtures may explode at 480
degree C. Generators of both types are usually designed to operate below 150
degree C and 15 psi (100 kPa).
The crude gas (containing traces of
Hydrogen Sulfide, Ammonia, and Phosphine) from the generator is either scrubbed
with water and Caustic Soda solution or Led to purifier where the impurities
are absorbed by the use of Iron oxide or active Chlorine compounds. The dry gas
is fed to cylinders to manufacturing units.
PROPERTIES
Colorless, Flammable gas, odorless when
pure but ordinarily has a Garlic like order because of impurities.
Molecular Weight : 26.04
Specific Gravity : 0.618, 20
degree C/4
Freezing Point : -80.8 degree C
Boiling Point : -84 degree C
Flash Point (open cup) : Gas
Ignition Temperature : 644 degree C
Vapor Density (air = 1) : 0.91
Explosive Limits % by volume in air : Lower 2.5 and Upper 82
Threshold Limit value in ppm : 1000
One pound of Acetylene is equivalent to 14.5 cubic ft (1 kg
= 0.9 cubic meter). Soluble in Acetone (2500 ml gas/100 g, 3000 ml gas/100 g at
12 atm), ethanol (600 ml/100 g), water (100 ml/100 g), and liquid a
Ammonia at room temperature.
Containers &
Regulations – Steel pressure cylinder containing
from 60 to 300 cubic ft of Acetylene (1.7 to 8.5 cubic meter). Mixtures of Air
and acetylene, as well as liquid Acetylene are highly explosive. The cylinder
must therefore contain a suitable solvent (such as Acetone) and generally a
porous material (such as Asbestos, kapok, or diatomaceous earth) on which the
solution is impregnated. The cylinder pressure must not exceed 250 psi (1.7
MPa) at 21 degree C, and requires a Red DOT shipping
label.
ECONOMIC ASPECTS
Acetylene is produced on the post where
needed by means of portable generators utilizing Calcium Carbide.
Economic Ascepts
Acetylene, Once used primarily in the
metal industry for cutting and welding, is now used chiefly in the manufacture
of chemicals. In this field economics problems of Acetylene manufacturer and
use are of dual nature: whether to use Acetylene at all in a given process and,
if so, whether to make it from Calcium Carbide or from Hydrocarbon gases.
The major chemicals presently
manufactured, at last in part, from acetylene are Vinyl Chloride monomer, Vinyl
Acetate, Acrylates, and tri and perchloroethylenes. Many of these can be made
alternatively from ethylene and other petroleum hydrocarbons, so ultimate costs
by the two routes becomes an important factor. Currently, ethylene is favored
for vinyl acetate and chloride; propylene is preferred for the manufacture of
Acrylates. Manufacturing routes to Acetaldehyde, Acetic acid, and
Acrylonitrile, formerly held by acetylene, have been taken over by Ethylene and
Propylene.
Increased use of Acetylene has developed through
Rappe chemicals. Some of these are propargyl Alcohol, Butyrolactone, Vinyl
pyrrolidone, Polyvinylpyrrolidone, 1,4 – butanediol, 2 – butyne – 1,4 – diol,
and Tetrahydrofuran.
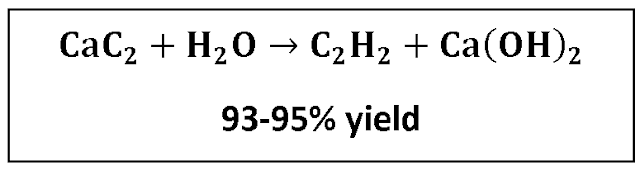
Comments
Post a Comment
If you have any doubts, please let me know